››Convert mole to atom
Z: Element: Ka 2 eV(unc) Ka 1 eV(unc) 10: Ne: 848.61(26) 848.61(26) 11: Na: 1040.98(12) 1040.98(12) 12: Mg: 1253.437(13) 1253.688(11) 13: Al: 1486.295(10) 1486.708(10.
- Magnesium adalah suatu unsur kimia dalam tabel periodik yang memiliki lambang Mg dan nomor atom 12. Ia berupa padatan abu-abu mengkilap yang memiliki kemiripan fisik dengan lima unsur lainnya pada kolom kedua (golongan 2, atau logam alkali tanah) tabel periodik: semua unsur golongan 2 memiliki konfigurasi elektron yang sama pada kelopak elektron terluar dan struktur kristal yang serupa.
- Mass particle = M (Mg) A, the particle being the one atom, so the mass for one atom. M(M g) = M (Mg) A = 24.305 g mol 6.02214086 ⋅ 1023 particle mol = 4.03594014 ⋅ 10−23 g particle So 4.03594014 ⋅ 10−23 grams is the mass per particle, the particle being an atom.
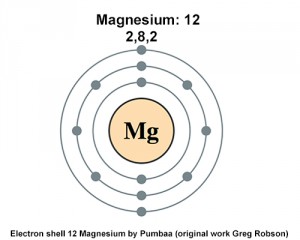
- (b) Atomic number and the number of electrons in magnesium atom is 12. So, electronic configuration is 2, 8, 2 (because 12=2 + 8 + 2). You can also Download NCERT Exemplar Class 9 Science Solutions to help you to revise complete Syllabus and score more marks in your examinations.
- The calculation was performed for an hcp sample (6 nm × 3 nm × 5 nm) consisting of 4000 atoms: one Al atom, one Ca (or Zn) atom, and 3998 Mg atoms. The binding energy was obtained by subtracting the total energy of the sample when the two solute atoms are distant from the total energy when these atoms are neighboring.

Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
››More information from the unit converter
How many moles in 1 atom?The answer is 1.660538863127E-24.
We assume you are converting between mole and atom.
You can view more details on each measurement unit:
moles oratom
The SI base unit for amount of substance is the mole.
1 mole is equal to 6.0221415E+23 atom.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between moles and atoms.
Type in your own numbers in the form to convert the units!
››Quick conversion chart of moles to atom
1 moles to atom = 6.0221415E+23 atom
2 moles to atom = 1.2044283E+24 atom
3 moles to atom = 1.80664245E+24 atom
4 moles to atom = 2.4088566E+24 atom
5 moles to atom = 3.01107075E+24 atom
6 moles to atom = 3.6132849E+24 atom
7 moles to atom = 4.21549905E+24 atom
8 moles to atom = 4.8177132E+24 atom
9 moles to atom = 5.41992735E+24 atom
10 moles to atom = 6.0221415E+24 atom
››Want other units?
You can do the reverse unit conversion fromatom to moles, or enter any two units below:
››Common amount of substance conversions
moles to centimol
moles to decimol
moles to picomol
moles to micromol
moles to nanomol
moles to millimol
moles to molecule
moles to kilomol
››Definition: Mole
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.'
››Definition: Atom
This site uses an exact value of 6.0221415 x 1023 for Avogadro's number. This is the number of atoms in 1 mole of a chemical element.
››Metric conversions and more
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!
››Convert mole to atom
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
››More information from the unit converter
How many moles in 1 atom?The answer is 1.660538863127E-24.
We assume you are converting between mole and atom.
You can view more details on each measurement unit:
moles oratom
The SI base unit for amount of substance is the mole.
1 mole is equal to 6.0221415E+23 atom.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between moles and atoms.
Type in your own numbers in the form to convert the units!
››Quick conversion chart of moles to atom


1 moles to atom = 6.0221415E+23 atom
2 moles to atom = 1.2044283E+24 atom
3 moles to atom = 1.80664245E+24 atom
4 moles to atom = 2.4088566E+24 atom
5 moles to atom = 3.01107075E+24 atom
6 moles to atom = 3.6132849E+24 atom
7 moles to atom = 4.21549905E+24 atom
8 moles to atom = 4.8177132E+24 atom
9 moles to atom = 5.41992735E+24 atom
10 moles to atom = 6.0221415E+24 atom
Mg Atom
››Want other units?
You can do the reverse unit conversion fromatom to moles, or enter any two units below:
Mg Atomic Number
››Common amount of substance conversions
moles to decimol
moles to centimol
moles to millimol
moles to micromol
moles to molecule
moles to picomol
moles to nanomol
moles to kilomol
››Definition: Mole
Mg Atom Drawing
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.'
››Definition: Atom
This site uses an exact value of 6.0221415 x 1023 for Avogadro's number. This is the number of atoms in 1 mole of a chemical element.
Mg Atom Size
››Metric conversions and more
Mg Atomic Mass
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!

